Research
A major focus of research in our group is the development of Textile-based advanced biomaterials, either as Medical Textiles, or as scaffolds having highly-controlled architectures and chemistries for developing three-dimensional cell culture and tissue regeneration. We are particularly interested in understanding how cells sense information from materials (architectures, compositions, surface features and mechanical properties) and based on that information how cells self-assemble to create three-dimensional microenvironment, how fibrous ECM components can be organized within the tissue construct by simulating anatomical orientation, to correlate with its biomechanics.
Based on these understanding we try to generate cellular grafts based on autologous cells and porous 3D scaffolds to repair cartilage, bone and muscle tissues, as well as complex tissue interfaces. Scaffolds of wide varieties of 3D architectures and mechanical properties will be designed by using concepts of Textile technology (knitting, weaving, nonwoven, braiding) or microfabrication techniques (miniaturized rapid prototyping). Aside from scaffold and appropriate cell source, environmental inputs - such as appropriate biomechanical forces, hydrodynamic fluid transport of nutrients and metabolic waste products and growth factors, are considered to develop in vitro engineered tissue constructs.
Beyond a potential clinical use as implants, these engineered constructs will also be considered as 3D tissue model systems, to investigate stem/progenitor cell differentiation and tissue development. Currently, pharmaceutical industries are in need of in vitro tissue model, using preferably human cells, to understand how drug candidates work and get mechanistic insights into the process. Successful developments of engineered tissue models, which simulate their in vivo counterparts, have great potential to advance our understanding of human disease processes, in terms of intra and inter-cellular signaling pathways in the context of 3D tissue microenvironment.
Our current research initiatives are focused on:
- Development of engineered tissue constructs (e.g., cartilage and bone-like tissues) to replace diseased or injured organs,
- Establishment of simple in vitro disease model system (especially for arthritis, degenerative Intervertebral disc) by microfabricating tissue equivalents with precise spatiotemporal and cellular microenvironments, using cells and Textile polymer based constructs (made up of rapid prototyping, weaving, knitting, nonwoven, Fibre-hydrogel composite).
- To design some 'smart' textile architectures for medical applications, such as Hernia mesh, post-surgical adhesion preventive barrier, etc.

- Tissue Engineering
- Cartilage & Bone Tissue Engineering
- Inter-vertebral disc regeneration
- Establishment of in vitro disease model system
- Medical Textiles
- Wound dressings
- Fibre-Hydrogel composite
- Hernia Mesh
- Sutures
- Polymeric Nano-materials
- Electrospinning
- Rapid Prototyping for making novel scaffolds for Tissue engineering
- Microfluidic chip-based Biosensors
- Bioreactors
- Dynamic culture condition for creating specific tissue microenvironment
Research Topics \ In vitro disease model systems
|
|
Beyond a potential clinical use as implants, the engineered constructs will also be considered as 3D tissue model systems, to investigate stem/progenitor cell differentiation and tissue development. Currently, pharmaceutical industries are in need of in vitro tissue model, using preferably human cells, to understand how drug candidates work and get mechanistic insights into the process. Successful developments of engineered tissue models, which simulate their in vivo counterparts, have great potential to advance our understanding of human disease processes, in terms of intra and inter-cellular signaling pathways in the context of 3D tissue microenvironment.
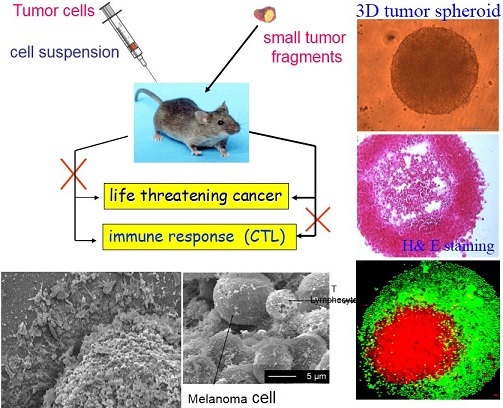
3D melanoma spheroid model to explore how 3D tumor architecture can cause lack of immune response
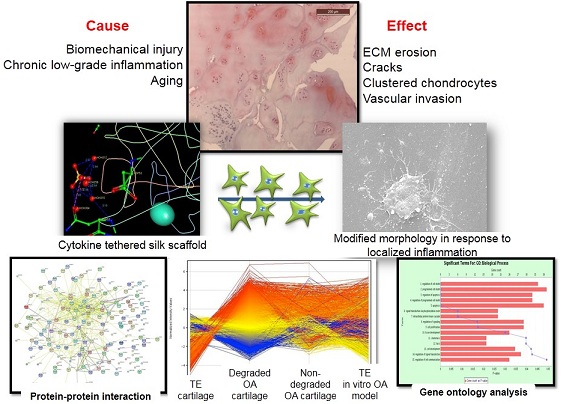
In vitro osteoarthritic cartilage model by culturing healthy human chondrocytes over a silk scaffold (having noncovalently immobilized inflammatory cytokines)
Publications
- Tissue Engineering Part A, 2013, 9, 1733-1753
- Trends in Molecular medicine, 2008, 14(8):333-340
- FEBS Letters. 2007; 581(23): 4523-28
- British Journal of Cancer, 2007, 96(7):1072-82
|


